BEL VISO MEDICAL SPA
IPL PHOTOREJUVENATION
Sclerotherapy
Take your legs out of hiding
Gain back the confidence to show off your legs with Asclera®
- Non-surgical: Administered in a provider’s office
- Reliable: FDA-approved in the U.S. since 2010
- Convenient: A quick return to most activities
- Satisfied Patients: 87% of clinical study patients were satisfied or very satisfied
- Side Effects: In a clinical study, the most common side effect was temporary bruising (hematoma) at the injection site*^
FDA APPROVED TO TREAT SPIDER AND RETICULAR VEINS IN THE LOWER EXTREMITIES
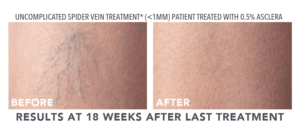
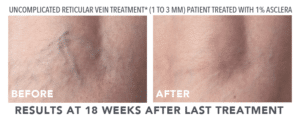
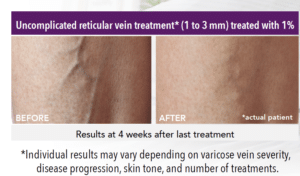
Look to Asclera® for Treatment of Spider Veins
If you’re one of the estimated 30 million Americans1 with venous disease, you’re not alon
The good news is that thanks to Asclera® (pronounced uh-SKLAIR-uh), you may no longer need to feel self-conscious about showing your legs due to spider veins. Asclera® is an FDA-approved treatment used in a procedure called sclerotherapy. It’s administered in-office at Bel Viso Medical Spa by a healthcare provider to treat two types of veins in the lower extremities.
- Uncomplicated spider veins (very small varicose veins ≤ 1 mm in diameter)
- Uncomplicated small varicose veins (1 to 3 mm in diameter) known as reticular
The Asclera® procedure
- Your provider or vascular specialist injects Asclera® into the vein.
- Over a period of weeks, the treated veins collapse and become less visible.
- Over time, the body naturally resorbs the treated vein.
*Asclera® Full Prescribing Information. Merz Aesthetics, 2019
* Rabe E, Schliephake D, Otto J, Breu FX, Pannier F. Sclerotherapy of telangiectases and reticular veins: a double-blind, randomized, comparative clinical trial of polidocanol, sodium tetradecyl sulphate and isotoicsaline (EASI study). Phlebology. 2010;25(3):124-131.